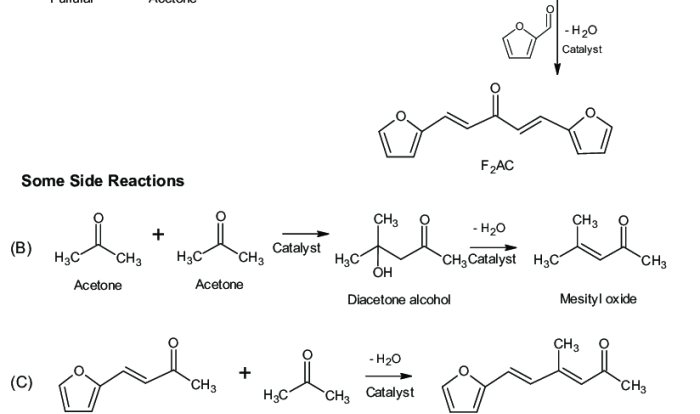
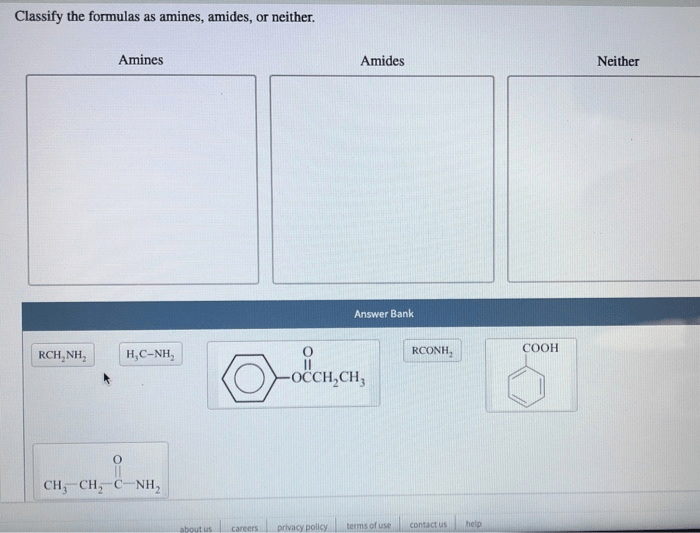
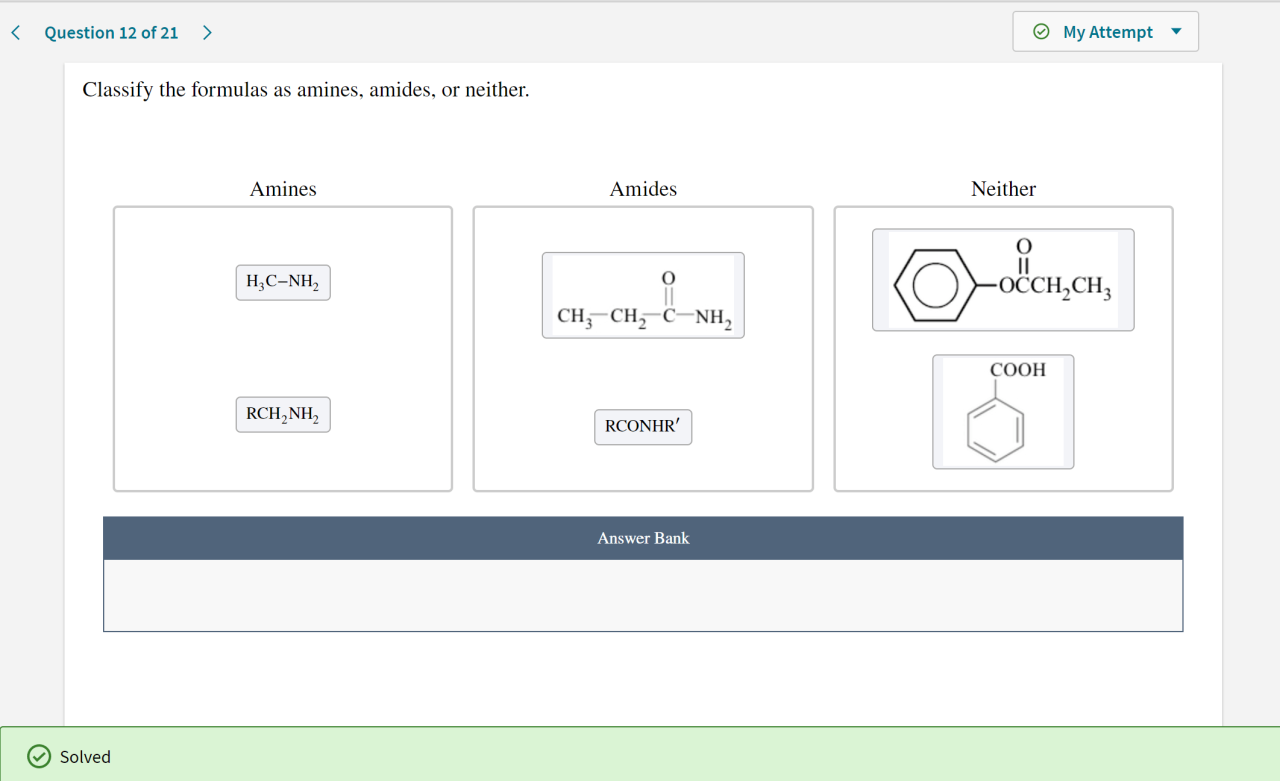
Classify the formulas as amines amides or neither – In the realm of chemistry, the classification of formulas as amines, amides, or neither holds immense significance. This comprehensive guide embarks on an exploration of the distinct characteristics, properties, and methods employed to differentiate these important functional groups. Join us as we delve into the intricacies of amines and amides, unraveling their chemical structures, reactivity patterns, and diverse applications.
From the fundamental definitions to advanced analytical techniques, this discourse provides a thorough understanding of the classification process. Prepare to expand your chemical knowledge and master the art of accurately identifying these essential compounds.
Definition and Overview of Amines and Amides
Aminesare organic compounds characterized by the presence of a nitrogen atom with at least one alkyl or aryl group attached. They have the general formula RNH 2, where R represents an alkyl or aryl group.
Amidesare organic compounds that contain a carbonyl group (C=O) bonded to a nitrogen atom. They have the general formula RCONH 2, where R represents an alkyl or aryl group.
The key difference between amines and amides lies in the hybridization of the nitrogen atom. In amines, the nitrogen atom is sp 3hybridized and has a lone pair of electrons, making it a Lewis base. In amides, the nitrogen atom is sp 2hybridized and does not have a lone pair of electrons, making it a Lewis acid.
Methods for Classifying Amines and Amides
Amines and amides can be classified using various methods, including chemical tests, spectroscopy, and advanced techniques.
Chemical Tests, Classify the formulas as amines amides or neither
- Hinsberg test:Distinguishes between primary, secondary, and tertiary amines based on their reactivity with benzenesulfonyl chloride.
- Hofmann test:Distinguishes between primary, secondary, and tertiary amides based on their reactivity with bromine in aqueous sodium hydroxide.
Spectroscopy
- Infrared (IR) spectroscopy:Identifies the presence of functional groups, such as the N-H bond in amines and the C=O bond in amides.
- Nuclear magnetic resonance (NMR) spectroscopy:Provides information about the structure and connectivity of atoms in a molecule, including the chemical environment of nitrogen atoms in amines and amides.
Examples of Amines and Amides
| Compound | Chemical Structure | IUPAC Name | Classification |
|---|---|---|---|
| Methylamine | CH3NH2 | Methanamine | Primary amine |
| Dimethylamine | (CH3)2NH | N,N-Dimethanamine | Secondary amine |
| Trimethylamine | (CH3)3N | N,N,N-Trimethylamine | Tertiary amine |
| Acetamide | CH3CONH2 | Ethanamide | Primary amide |
| Benzamide | C6H5CONH2 | Benzamide | Primary amide |
Properties and Applications:
- Amines are often basic and can be used as solvents, catalysts, and drugs.
- Amides are generally neutral and are used as solvents, plasticizers, and in the production of synthetic fibers.
Classification of Formulas
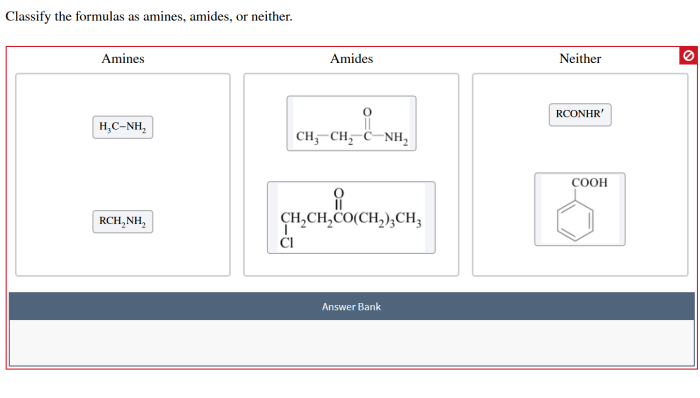
Formulas can be classified as amines, amides, or neither based on the following criteria:
- Presence of a nitrogen atom:All amines and amides contain a nitrogen atom.
- Hybridization of the nitrogen atom:Amines have sp 3hybridized nitrogen atoms, while amides have sp 2hybridized nitrogen atoms.
- Presence of a carbonyl group:Amides contain a carbonyl group (C=O) bonded to the nitrogen atom, while amines do not.
Flowchart for Classification:
- Does the formula contain a nitrogen atom?
- Yes: Go to step 2.
- No: Not an amine or amide.
- Is the nitrogen atom bonded to a carbonyl group (C=O)?
- Yes: Amide.
- No: Go to step 3.
- Is the nitrogen atom sp3hybridized?
- Yes: Amine.
- No: Not an amine or amide.
Common Errors and Pitfalls:
- Confusing imines (R 2C=NH) with amines (RNH 2).
- Classifying compounds with nitrogen atoms in other functional groups (e.g., nitriles, nitro compounds) as amines or amides.
Advanced Techniques for Classification
Advanced techniques, such as mass spectrometry and X-ray crystallography, can be used to resolve complex classification problems.
Mass Spectrometry
Mass spectrometry can provide information about the molecular weight and fragmentation patterns of a compound, which can help in identifying its structure and classifying it as an amine or amide.
X-ray Crystallography
X-ray crystallography can provide detailed information about the molecular structure of a compound, including the hybridization of the nitrogen atom and the presence of a carbonyl group, which can be used to classify it as an amine or amide.
Advantages and Limitations:
- Advantages:Advanced techniques can provide definitive information about the structure and classification of a compound.
- Limitations:These techniques can be expensive and time-consuming, and they may not be suitable for all samples.
FAQ Overview: Classify The Formulas As Amines Amides Or Neither
What is the key difference between an amine and an amide?
Amines possess a nitrogen atom bonded to two hydrogen atoms, while amides have a nitrogen atom double-bonded to a carbon atom and single-bonded to a hydrogen atom.
How can I use spectroscopy to classify amines and amides?
Infrared (IR) and nuclear magnetic resonance (NMR) spectroscopy provide valuable information about the functional groups present in a molecule, aiding in the classification process.
What are some common errors in classifying formulas as amines, amides, or neither?
Mistaking imines for amides, overlooking the presence of resonance structures, and misinterpreting the hybridization of nitrogen atoms are common pitfalls to avoid.